FINAL EVENT of the Interreg 2 Seas project “Site-Specific Drug delivery”
This event was held online on the 30th of March 2023. More than 120 participants from all around the world (>20 countries) had registered: from the industry, academia and regulatory authorities.
Key results of the project were presented by the differents partners, including:
Parenteral, biodegradable drug delivery systems: Importance of the in vitro release set-up
Prof. Juergen Siepmann, University of Lille, France
Advanced drug delivery systems for colon targeting
Dr. Atheer Awad, University College London, UK
Innovative implants for inner ear treatments
Dr. Julian Apachitei, University of Delft, The Netherlands
Drug loaded breast implants
Dr. Kevin Roux and Jaime Destouesse, Lattice Medical, Loos, France
Cutting edge in vitro characterization techniques for local drug delivery systems
Prof. Axel Zeitler, University of Cambridge, UK
Towards more realistic drug release measurements
Dr. Frédéric Moens, ProDigest, Belgium

4th European Conference on Pharmaceutics
Marseille, France, 20 & 21 March 2023
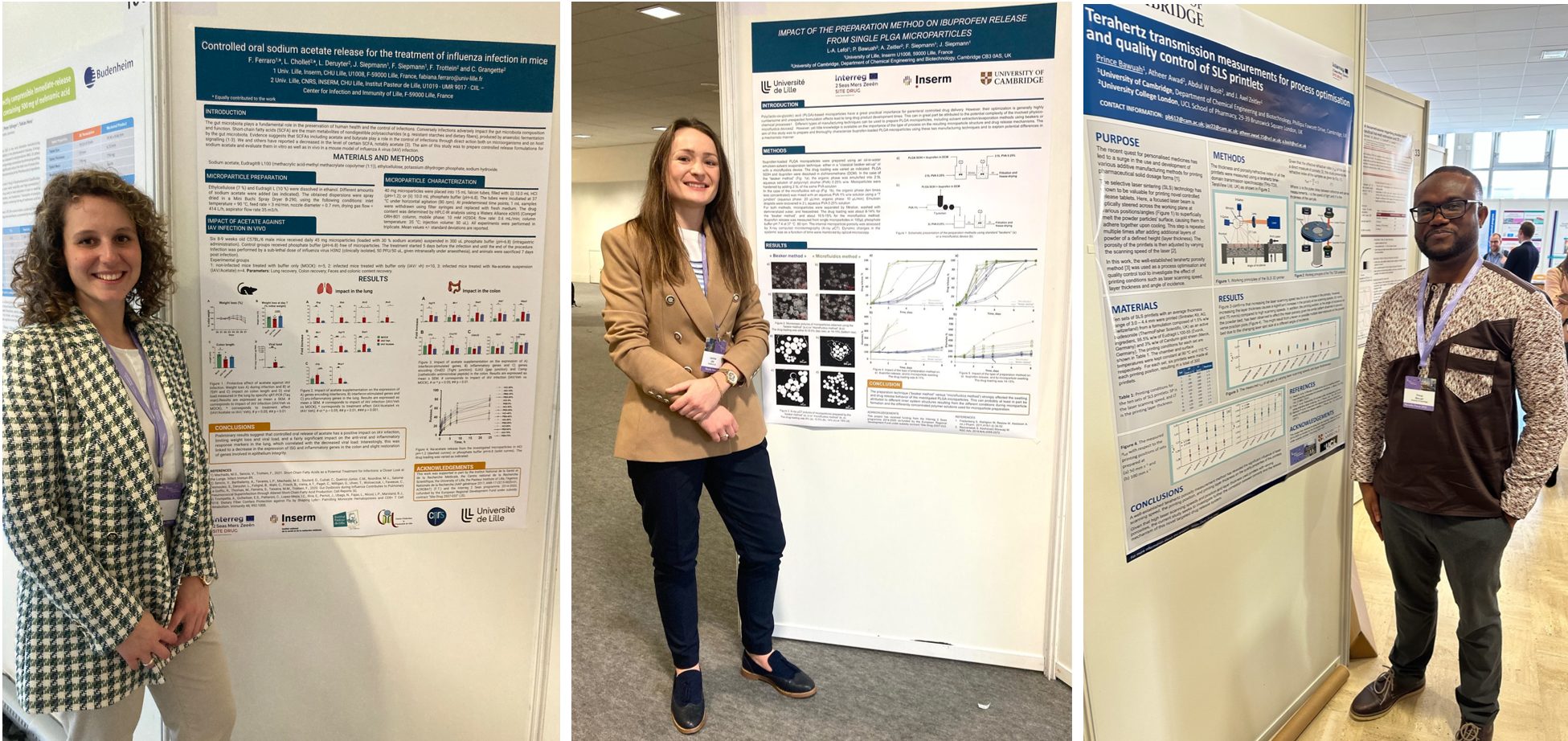
The Interreg project Site Drug was exhibitor at the 4th European Conference on Pharmaceutics, which was held on 20 & 21 March 2023 in Marseille, France.
The conference was dedicated to “Advanced technologies enabling new therapies“ and attracted more than 550 participants working in the field of drug delivery.
The conference offered the opportunity to initiate fruitful exchange and cooperation.

The meeting was also the opportunity to present the latest results of the Interreg Site Drug project either as oral and poster presentations.
Special issue: International Journal of Pharmaceutics: X

Parts of the results obtained in this Interreg project are published in a special issue of the gold open access journal IJPX:
Please have a look at the articles for more detailed information on the findings of this project. So fra, 6 articles have been published, more are to come…
4 Site Drug Researchers recognized as
“Highly Cited Researchers 2022”
Clarivate recently announced the names of the “Highly Cited Researchers 2022”:

Professor Abdul Basit, Professor Simon Gaisford and Dr Alvaro Goyanes of the UCL School of Pharmacy have received the honour of being Highly Cited Researchers for a fourth year in a row. Excitingly, Dr. Atheer Awad, young researcher joined the Highly Cited 2022 list. It is a remarkable achievement for a young scientist to appear on this prestigious list. They are all working on innovative drug delivery systems for colon targeting in the frame of the Site Drug project.
“Out of nearly 8 Million researchers in the world over the last decade, less than 1% have published multiple papers frequently cited by their peers that rank in the top 1% of citations for field and year. Since 2001, our experts have used Web of Science™ citation data to identify these very influential researchers.” (Clarivate @ https://clarivate.com) |  |
Read our latests publications
Visualising liquid transport through coated pharmaceutical tablets using Terahertz pulsed imaging
R. Dong, J. A. Zeitler
International Journal of Pharmaceutics, Volume 619, 2022, 121703,
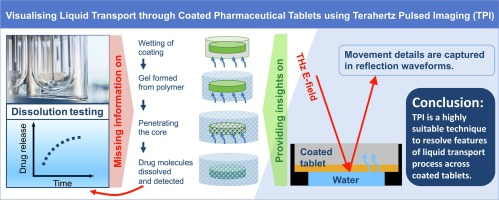
Dissolution of pharmaceutical tablets is a complex process, especially for coated tablets where layered structures form an additional barrier for liquid transport into the porous tablet matrix. A better understanding of the role of the coating structure in the mass transport processes that govern drug release, starting with the wetting of the coating layer by the dissolution medium, can benefit the formulation design and optimisation of the production. For this study, terahertz pulsed imaging was used to investigate how dissolution medium can penetrate coated tablets. In order to focus on the fundamental process, the model system for this proof-of-principle study consisted of tablet cores made from pure microcrystalline cellulose compacted to a defined porosity coated with Opadry II, a PVA-based immediate release coating blend. The coating was applied to a single side of flat-faced tablets using vacuum compression moulding. It was possible to resolve the hydration of the coating layer and the subsequent liquid ingress into the dry tablet core. The analysis revealed a discontinuity in density at the interface between coating and core, where coating polymer could enter the pore space at the immediate surface of the tablet cores during the coating process. This structure affected the liquid transport of the dissolution medium into the core. We found evidence for the formation of a gel layer upon hydration of the coating polymer. The porosity of the tablet core impacted the quality of coating and thus affected its dissolution performance (r = 0.6932 for the effective liquid penetration rate RPeff and the core porosity). This study established a methodology and can facilitate a more in-depth understanding of the role of coating on tablet dissolution.
Reprinted from International Journal of Pharmaceutics, Volume 619, 2022, 121703, Dong et al. Visualising liquid transport through coated pharmaceutical tablets using Terahertz pulsed imaging, https://doi.org/10.1016/j.ijpharm.2022.121703 under the Creative Commons CC BY license.
The Dynamic Intestinal Absorption Model (Diamod®), an in vitro tool to study the interconnected kinetics of gastrointestinal solubility, supersaturation, precipitation, and intestinal permeation processes of oral drugs
Frédéric Moens, Gies Vandevijver, Anke De Blaiser, Adam Larsson, Fabio Spreafico, Patrick Augustijns, Massimo Marzorati
International Journal of Pharmaceutics: X, Volume 5, 2023, 100177
https://doi.org/10.1016/j.ijpx.2023.100177
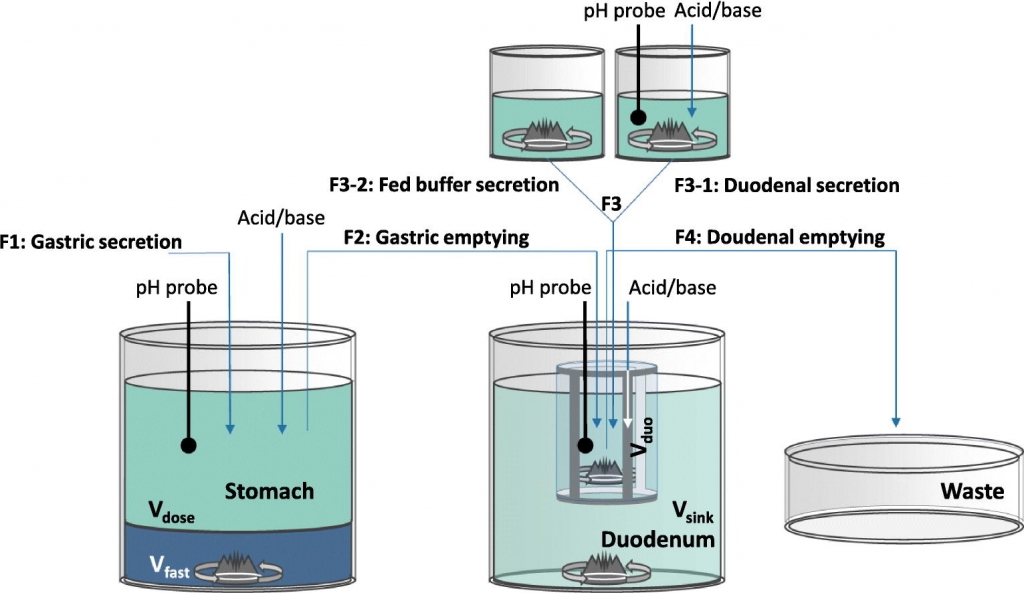
This study aimed at developing the Diamod® as a dynamic gastrointestinal transfer model with physically interconnected permeation. The Diamod® was validated by studying the impact of the intraluminal dilution of a cyclodextrin-based itraconazole solution and the negative food effect for indinavir sulfate for which clinical data are available demonstrating that the systemic exposure was strongly mediated by interconnected solubility, precipitation, and permeation processes. The Diamod® accurately simulated the impact of water intake on the gastrointestinal behavior of a Sporanox® solution. Water intake significantly decreased the duodenal solute concentrations of itraconazole as compared to no intake of water. Despite this duodenal behavior the amount of permeated itraconazole was not affected by water intake as observed in vivo. Next to this, the Diamod® accurately simulated the negative food effect for indinavir sulfate. Different fasted and fed state experiments demonstrated that this negative food effect was mediated by an increased stomach pH, entrapment of indinavir in colloidal structures and the slower gastric emptying of indinavir under fed state conditions. Therefore, it can be concluded that the Diamod® is a useful in vitro model to mechanistically study the gastrointestinal performance of drugs.
Reprinted from International Journal of Pharmaceutics: X, Volume 3, 2022, 100177, Moens et al. The Dynamic Intestinal Absorption Model (Diamod®), an in vitro tool to study the interconnected kinetics of gastrointestinal solubility, supersaturation, precipitation, and intestinal permeation processes of oral drugs, https://doi.org/10.1016/j.ijpx.2023.100177, under the Creative Commons CC BY 4.0 License.
Detecting Crystallinity Using Terahertz Spectroscopy in 3D Printed Amorphous Solid Dispersions
S. Santitewagun, R.Thakkar, J. A. Zeitler and M. Maniruzzaman
Mol. Pharmaceutics 2022, 19, 7, 2380–2389
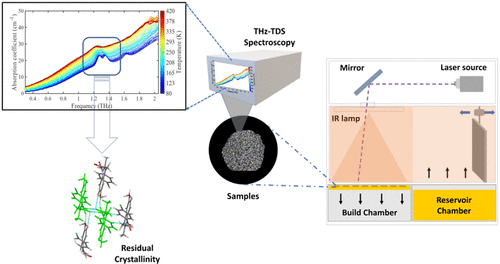
This study demonstrates the applicability of terahertz time-domain spectroscopy (THz-TDS) in evaluating the solid-state of the drug in selective laser sintering-based 3D printed dosage forms. Selective laser sintering is a powder bed-based 3D printing platform, which has recently demonstrated applicability in manufacturing amorphous solid dispersions (ASDs) through a layer-by-layer fusion process. When formulating ASDs, it is critical to confirm the final solid state of the drug as residual crystallinity can alter the performance of the formulation. Moreover, SLS 3D printing does not involve the mixing of the components during the process, which can lead to partially amorphous systems causing reproducibility and storage stability problems along with possibilities of unwanted polymorphism. In this study, a previously investigated SLS 3D printed ASD was characterized using THz-TDS and compared with traditionally used solid-state characterization techniques, including differential scanning calorimetry (DSC) and powder X-ray diffractometry (pXRD). THz-TDS provided deeper insights into the solid state of the dosage forms and their properties. Moreover, THz-TDS was able to detect residual crystallinity in granules prepared using twin-screw granulation for the 3D printing process, which was undetectable by the DSC and XRD. THz-TDS can prove to be a useful tool in gaining deeper insights into the solid-state properties and further aid in predicting the stability of amorphous solid dispersions.
Reprinted with permission from Mol. Pharmaceutics 2022, 19, 7, 2380–2389. Santitewagun et al., Detecting Crystallinity Using Terahertz Spectroscopy in 3D Printed Amorphous Solid Dispersions https://doi-org.ressources-electroniques.univ-lille.fr/10.1021/acs.molpharmaceut.2c00163. Copyright 2022, American Chemical Society.”
Dexamethasone-loaded cochlear implants: How to provide a desired “burst release”,
A. Qnouch, V. Solarczyk, J. Verin, G. Tourrel, P. Stahl, F. Danede, J.F. Willart, P.E. Lemesre, C. Vincent, J. Siepmann, F. Siepmann
International Journal of Pharmaceutics: X, Volume 3, 2021, 100088.
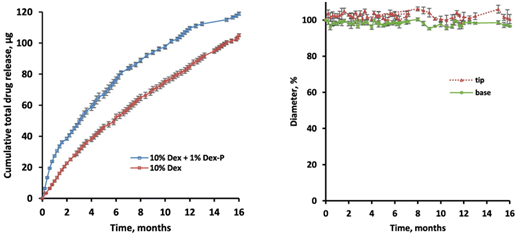
Cochlear implants containing iridium platinum electrodes are used to transmit electrical signals into the inner ear of patients suffering from severe or profound deafness without valuable benefit from conventional hearing aids. However, their placement is invasive and can cause trauma as well as local inflammation, harming remaining hair cells or other inner ear cells. As foreign bodies, the implants also induce fibrosis, resulting in a less efficient conduction of the electrical signals and, thus, potentially decreased system performance. To overcome these obstacles, dexamethasone has recently been embedded in this type of implants: into the silicone matrices separating the metal electrodes (to avoid short circuits). It has been shown that the resulting drug release can be controlled over several years. Importantly, the dexamethasone does not only act against the immediate consequences of trauma, inflammation and fibrosis, it can also be expected to be beneficial for remaining hair cells in the long term. However, the reported amounts of drug released at “early” time points (during the first days/weeks) are relatively low and the in vivo efficacy in animal models was reported to be non-optimal. The aim of this study was to increase the initial “burst release” from the implants, adding a freely water-soluble salt of a phosphate ester of dexamethasone. The idea was to facilitate water penetration into the highly hydrophobic system and, thus, to promote drug dissolution and diffusion. This approach was efficient: Adding up to 10% dexamethasone sodium phosphate to the silicone matrices substantially increased the resulting drug release rate at early time points. This can be expected to improve drug action and implant functionality. But at elevated dexamethasone sodium phosphate loadings device swelling became important. Since the cochlea is a tiny and sensitive organ, a potential increase in implant dimensions over time must be limited. Hence, a balance has to be found between drug release and implant swelling.
Reprinted from International Journal of Pharmaceutics: X, Volume 3, 2022, 100088, Qnouch et al. Dexamethasone-loaded cochlear implants: How to provide a desired “burst release”, https://doi.org/10.1016/j.ijpx.2021.100088, under the Creative Commons CC BY NC ND License.