
Come to our “Site Drug” booth at the upcoming 4th European Conference on Pharmaceutics in Marseille – 20 & 21 March 2023!

The conference will be dedicated to “Advanced technologies enabling new therapies“.
The European Conferences on Pharmaceutics are held every two years and are jointly organized by the A.D.R.I.T.E.L.F. (“Italian Association of Pharmaceutical Technology and Law”), APGI (“International Society of Drug Delivery Sciences and Technology“) and APV (“International Association for Pharmaceutical Technology“).
The aim is to help bridging the gap between fundamental academic research and industrial applications, offering the opportunity to initiate fruitful exchange and cooperation between university and industry. The conference will be a perfect occasion for young as well as for established scientists from academia and industry from all over the world to present their work, network, discuss newest scientific findings and to share their experience with colleagues.
For more information on the 4th European Conference on Pharmaceutics please have a look at: https://www.europeanmeeting.org/home
Dr Atheer Awad was named in Forbes 30 Under 30 list 2022 European list in the Science and Healthcare category

Forbes is a global media company, focusing on business, investing, technology, entrepreneurship, leadership, and lifestyle. Their Under 30 list recognises people under 30 years old and is issued annually.
The European lists recognise 300 business and industry figures, with 30 selected in 10 industries each.
Links: https://www.forbes.com/30-under-30/2022/europe/science-healthcare?profile=atheer-awad
Read our latests publications
Silicone matrices for controlled dexamethasone release: Towards a better understanding of the underlying mass transport mechanisms
T. Rongthong, A. Qnouch, M. Maue Gehrke, L. Paccou, P. Oliveira, F. Danede, J. Verin, C. Vincent, J. F. Willart, F. Siepmann, J. Siepmann
Regenerative Biomaterials, 2023, rbad008
https://doi.org/10.1093/rb/rbad008
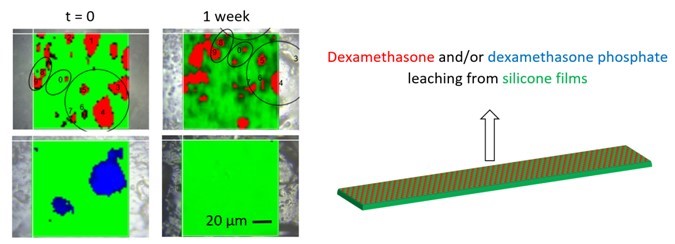
Dexamethasone-loaded silicone matrices offer an interesting potential as innovative drug delivery systems, e.g. for the treatment of inner ear diseases or for pacemakers. Generally, very long drug release periods are targeted: several years/decades. This renders the development and optimization of novel drug products cumbersome: Experimental feedback on the impact of the device design is obtained very slowly. A better understanding of the underlying mass transport mechanisms can help facilitating research in this field. A variety of silicone films was prepared in this study, loaded with amorphous or crystalline dexamethasone. Different polymorphic drug forms were investigated, the film thickness was altered and the drug optionally partially/completely exchanged by much more water-soluble dexamethasone phosphate. Drug release studies in artificial perilymph, SEM, optical microscopy, DSC, X-ray diffraction and Raman imaging were used to elucidate the physical states of the drugs & polymer, and of the systems’ structure as well as dynamic changes thereof upon exposure to the release medium. Dexamethasone particles were initially homogeneously distributed throughout the systems. The hydrophobicity of the matrix former very much limits the amounts of water penetrating into the system, resulting in only partial drug dissolution. The mobile drug molecules diffuse out into the surrounding environment, due to concentration gradients. Interestingly, Raman imaging revealed that even very thin silicone layers (<20 µm) can effectively trap the drug for prolonged periods of time. The physical state of the drug (amorphous, crystalline) did not affect the resulting drug release kinetics to a noteworthy extent.
Reprinted from Regenerative Biomaterials, 2023; rbad008, Rongthong et al., Silicone matrices for controlled dexamethasone release: Towards a better understanding of the underlying mass transport mechanisms , https://doi.org/10.1093/rb/rbad008 under the CC BY license.
3D printed infliximab suppositories for rectal biologic delivery
A. Awad, A. Goyanes, M. Orlu, S.n Gaisford, A. W. Basit
International Journal of Pharmaceutics: X, 2023, 100176
https://doi.org/10.1016/j.ijpx.2023.100176
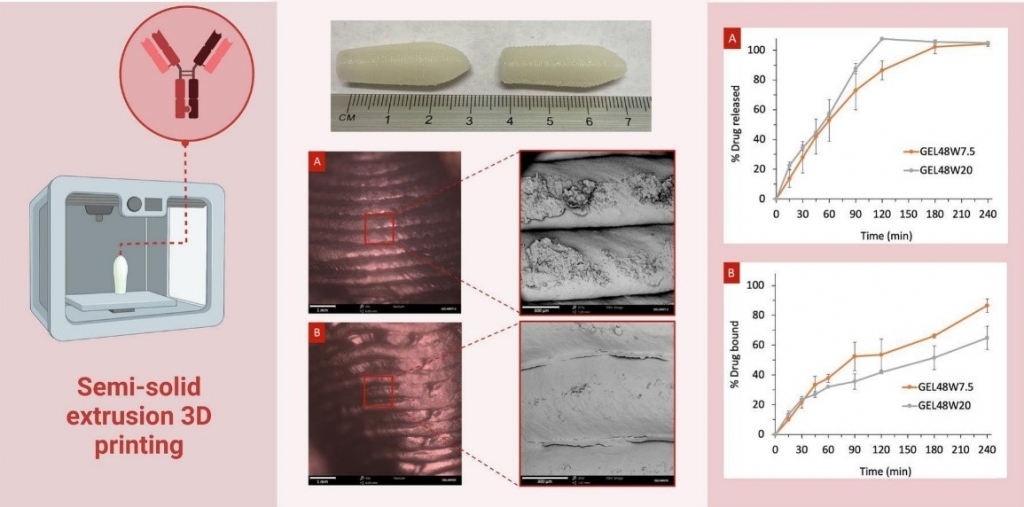
Infliximab is a monoclonal antibody that plays an important role in the management and treatment of chronic inflammatory bowel diseases. Due to its macromolecular structure, its delivery through the oral route is challenging, limiting its administration to only via the parenteral route. The rectal route offers an alternative way for administering infliximab, allowing it to be localised at the disease site and circumventing its passage across the alimentary canal and thus, maintaining its integrity and bioactivity. Three-dimensional (3D) printing is an advanced production technology that permits the creation of dose-flexible dosage forms from digital designs. The current study assessed the feasibility of utilising semi-solid extrusion 3D printing for the fabrication of infliximab-loaded suppositories for the local treatment of IBD. Various printing inks composed of Gelucire® (48/16 or 44/14) mixed with coconut oil and/or purified water were investigated. It was shown that following reconstitution in water, the infliximab solution can be directly incorporated into the printing ink of Gelucire® 48/16 and can withstand the extrusion process, resulting in well-defined suppositories. Since water content and temperature are critical for safeguarding infliximab’s potency, the effect of changing the composition of the printing inks and printing parameters on infliximab’s biologic efficiency was evaluated by measuring its binding capacity (i.e., the amount of infliximab that actively binds to its antigen to exert an effect). Despite drug loading assays showing that infliximab remains intact following printing, it was found that the incorporation of water in isolation results in only ~65% binding capacity. However, when oil is added to the mixture, infliximab’s binding capacity increases up to ~85%. These promising results demonstrate that 3D printing has the potential to be exploited as a novel platform for fabricating dosage forms containing biopharmaceuticals, avoiding patients’ compliance issues observed with injectables and addressing their unmet needs.
Reprinted from International Journal of Pharmaceutics: X, 2023, 100176, Awad et al. 3D printed infliximab suppositories for rectal biologic delivery, https://doi.org/10.1016/j.ijpx.2023.100176 under the Creative Commons CC BY license.
