
Read our latest publication in International Journal of Pharmaceutics: X
Long term behavior of dexamethasone-loaded cochlear implants: In vitro & in vivo
T. Rongthong, A. Qnouch, M. Maue Gehrke, F. Danede, J.F. Willart, P.F.M. de Oliveira, L. Paccou, G. Tourrel, P. Stahl, J. Verin, P. Toulemonde, C. Vincent, F. Siepmann, J. Siepmann
Int.J. Pharm. X, Volume 4, December 2022, 100141
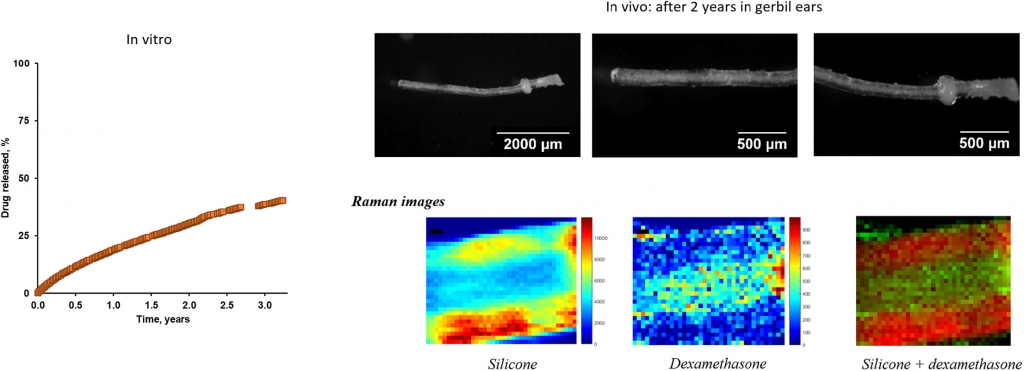
The aim of this study was to better understand the long term behavior of silicone-based cochlear implants loaded with dexamethasone: in vitro as well as in vivo (gerbils). This type of local controlled drug delivery systems offers an interesting potential for the treatment of hearing loss. Because very long release periods are targeted (several years/decades), product optimization is highly challenging. Up to now, only little is known on the long term behavior of these systems, including their drug release patterns as well as potential swelling or shrinking upon exposure to aqueous media or living tissue. Different types of cylindrical, cochlear implants were prepared by injection molding, varying their dimensions (being suitable for use in humans or gerbils) and initial drug loading (0, 1 or 10%). Dexamethasone release was monitored in vitro upon exposure to artificial perilymph at 37 °C for >3 years. Optical microscopy, X-ray diffraction and Raman imaging were used to characterize the implants before and after exposure to the release medium in vitro, as well as after 2 years implantation in gerbils. Importantly, in all cases dexamethasone release was reliably controlled during the observation periods. Diffusional mass transport and limited drug solubility effects within the silicone matrices seem to play a major role. Initially, the dexamethasone is homogeneously distributed throughout the polymeric matrices in the form of tiny crystals. Upon exposure to aqueous media or living tissue, limited amounts of water penetrate into the implant, dissolve the drug, which subsequently diffuses out. Surface-near regions are depleted first, resulting in an increase in the apparent drug diffusivity with time. No evidence for noteworthy implant swelling or shrinkage was observed in vitro, nor in vivo. A simplified mathematical model can be used to facilitate drug product optimization, allowing the prediction of the resulting drug release rates during decades as a function of the implant’s design.
The article is available in open access: click here

Dr Atheer Awad was recognised by MIT Technology Review
Dr Atheer Awad was recognised by MIT Technology Review as one of 15 Innovators Under 35 in MENA region.
Innovators Under 35 is an annual list that recognises outstanding innovators who are younger than 35. The search is for individuals whose superb technical work promises to shape the coming decades.
Dr Atheer Awad was recognised by MIT Technology Review as one of 15 Innovators Under 35 in MENA region.
Since 1999, MIT Technology Review has honoured the young innovators whose inventions and research are most exciting. Today that collection is Innovators Under 35, a list of technologists and scientists, all under the age of 35. Their work – spanning biomedicine, computing, communications, energy, materials, software, transportation, web and internet, and more – is changing the world!
The goal is to recognise the development of new technology or the creative application of existing technologies to solve the world’s biggest problems. IU35 rewards ingenious and elegant work that matters to the world at large — not just to peers in a particular field or industry. A panel of judges – experts in different fields – assess candidates, seeking a mixture of individuals that represent current trends in technology and the diversity of innovation around the globe. They seek innovators who introduce new and better solutions that change the way people live or work.

Professor Abdul Basit has been named as the recipient of the Harrison Memorial Medal for 2022
Professor Abdul Basit has been named as the recipient of the Harrison Memorial Medal for 2022. The Harrison Memorial Medal is awarded every two years, in memory of the distinguished pharmaceutical chemist Colonel EF Harrison. It is presented to an individual who has made an outstanding contribution in advancing pharmaceutical science.
Professor Basit is internationally recognised for leading in the fields of oral drug delivery, microbiome medicines, three-dimensional printing of pharmaceuticals and digital health. He has also founded three start-up companies.
His research has led to a series of transformative drug delivery systems, translated into the design of new technologies and improved therapies, many of which have been commercialised and launched worldwide including a new treatment for inflammatory bowel disease. To date, more than a million patients have benefited from inventions created and developed in the Basit Research Group.
Link: https://www.rpharms.com/about-us/news/details/rps-announces-harrison-memorial-medal-winner
